-
Water trapped in dibenzo-18-crown-6: theoretical and spectroscopic (IR, Raman) studies
M. Dulak, R. Bergougnant, K.M. Fromm, H. Hagemann, A.Y. Robin and T.A. Wesolowski
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 64 (2) (2006), p532-548


DOI:10.1016/j.saa.2005.07.060 | unige:3646 | Abstract | Article HTML | Article PDF
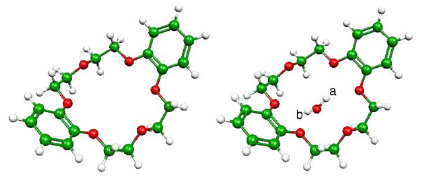
Experimental (IR and Raman) and theoretical (Kohn-Sham calculations) methods are used in a combined analysis aimed at refining the available structural data concerning the molecular guests in channels formed by stacked dibenzo-18-crown-6 (DB18C6) crown ether. The calculations are performed for a simplified model comprising isolated DB18C6 unit and its complexes with either H2O or H3O+ guests, which are the simplest model ingredients of a one-dimensional diluted acid chain, to get structural and energetic data concerning the formation of the complex and to assign the characteristic spectroscopic bands. The oxygen centers in the previously reported crystallographic structure are assigned to either H2O or protonated species.